【Introduction】
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases. Early detection of infected people is crucial to stopping the spread of this disease.
【Intended use】
Novel Coronavirus (SARS-CoV-2) Antigen Rapid Test (Colloidal Gold) is an in-vitro qualitative detection kit for the antigen of novel coronavirus presented in human Oropharyngeal swabs, Anterior Nasal swabs, or Nasopharyngeal swabs. This test kit is intended for use only by healthcare and laboratory professionals for the early diagnosis of patients with clinical symptoms of SARS-COV-2 infection.
The test kit can be used in any environment that meets the requirements of the instructions and local regulations. This test only provides preliminary test results. Negative results cannot exclude SARS-COV-2 infection, and they must be combined with clinical observation, history and epidemiological information. The result of this test should not be the sole basis for the diagnosis; confirmatory testing is required.
【Test principle】
This test kit adopts colloidal gold immunochromatography technology. When the specimen extraction solution moves forward along the test strip from the specimen hole to the absorbent pad under capillary action, If the specimen extraction solution contains novel coronaviruses antigen, the antigen will bind to the colloidal gold-labeled with anti-novel coronavirus monoclonal antibody, to form an immune complex. Then the immune complex will be captured by another anti-novel coronavirus monoclonal antibody, which is fixed in nitrocellulose membrane. A colorful line will appear in the test line “T” region, indicating novel coronavirus antigen positive; If test line “T” does not show color, a negative result will be obtained.
The test cassette also contains a quality control line “C”, which shall appear regardless of whether there is a visible T line.
【Main components】
1) Sterilized disposable virus sampling swab
2) Extraction tube with Nozzle Cap and extraction buffer
3) Test cassette
4) Instruction for Use
5) Biohazardous waste bag
【Storage and stability】
1.Store at 4~30℃ out of direct sunlight, and it is valid for 24 months from Production Date.
2.Keep dry, and do not use frozen and expired devices.
3.Test cassette should be used within half 1 hour once opening the Aluminum foil pouch.
【Warning and Precaution】
1.This kit is for in vitro detection only. Please use the kit within the validity period.
2.The Test is intended to aid in the diagnosis of a current COVID-19 infection. Please consult a healthcare professional to discuss your results and if any additional testing is required.
3.Please store the kit as the IFU shows, and avoid long period freezing conditions.
4.Read and follow the instructions carefully before using the kit, or an inaccurate result may be contained.
5.Do not replace the components from one kit to another.
6.Guard against moisture, do not open the aluminum platinum bag before it is ready for testing. Do not use the aluminum foil bag when it is found open.
7.All components of this kit should be placed in Biohazardous waste bag and be disposed according to the local require.
8.Avoid dumping, splashing.
9.Keep test kit and materials out of the reach of children and pets before and after use.
10.Make sure there is sufficient light when testing
11.Do not drink or dispose the antigen extraction buffer to your skin.
12.Children under 18 should be tested or guided by an adult.
13.Excess blood or mucus on the swab specimen may interfere with performance and may yield a false positive result.
【Specimen Collection and preparation】
Specimen collection:
Anterior Nasal swab
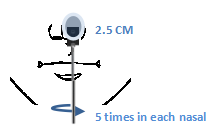
1.Insert the entire collection tip of the swab provided inside the nostril.
2.Firmly sample the nasal wall by rotating the swab in a circular path against the nasal wall at least 4 times.
3.Take approximately 15 seconds to collect the specimen. Be sure to collect any nasal drainage that may be present on the swab.
4.Repeat in the other nostril using the same swab.
5.Slowly remove the swab.
Specimen solution preparation:
1.Peel open the Sealing membrane in the Extraction tube.
2.Insert the fabric tip of the swab into the extraction buffer on the bottle of the tube.
3.Stir and Press the swab head against the extraction tube wall to release the antigen, rotating the swab for 1 minute.
4.Remove the swab while pinch the extraction tube against it.
(Make sure as much liquid in the fabric tip of the swab are removed as possible).
5.Press the Nozzle Cap provided tightly onto the extraction tube to avoid any possible leaks.
6.Dispose of swabs to biohazard waste bag.

Blow nose
Wash hands
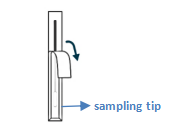
Get swab
Collect sample
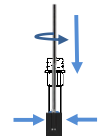
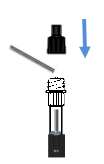
Insert,Press and Rotate the swab
Break off the swab and Replace the cap
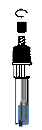
Unscrew the transparent cap
The specimen solution can keep stable for 8 hours at 2~8℃,3 hours at room temperature(15 ~ 30℃). Avoid more than four times of repeated freezing and thawing.
【Test procedure】
Do not open the pouch until you are ready to perform a test, and the test is suggested to conduct in room temperature (15 ~ 30℃),and avoid the extreme humid environment.
1.Remove the test cassette from the foil pouch and place it on a clean dry horizontal surface.
2.Upside down the extraction tube, put three drops into the specimen hole on the bottom of test cassette, and start the timer.
3.Waite and read the results in 15~25 minutes. Results before 15 minutes and after 25 minutes are invalid.
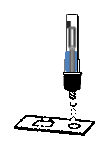

Add specimen solution
Read result at 15~25 min
【Interpretation of the test result】
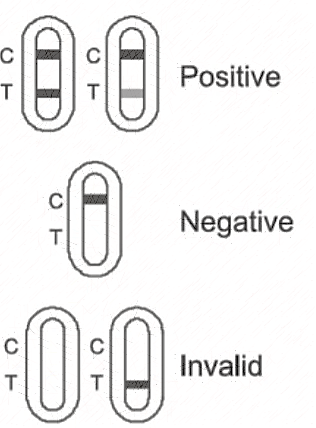
Negative result: If quality control line C appears, but the test line T is colorless, the result is negative, indicating no Novel Coronavirus antigen has been detected.
Positive results: If both quality control line C and test line T appear, the result is positive,indicating the Novel Coronavirus antigen has been detected.
Invalid result: If there is no quality control line C, whether the test line T appears or not, it indicates that the test is invalid and the test shall be repeated.
【Limitations】
1.This reagent is only used for qualitative detection and cannot indicate the level of novel coronavirus antigen in the specimen.
2.Due to the limitation of the detection method, the negative result cannot exclude the possibility of infection. The positive result should not be taken as a confirmed diagnosis. Judgement should be made along with clinical symptoms and further diagnosis methods.
3.In the early stage of infection, the test result may be negative because the low SARS-CoV-2 antigen level in the sample.
4.The accuracy of the test depends on the specimen collection and preparation process. Improper collection, transportation storage or freezing and thawing will affect the test results.
5.The volume of buffer added when eluted the swab are too much, non- standardized elution operation, low virus titer in the sample, these may all lead to false negative results.
6.It is optimum when eluting swabs with the matched antigen extraction buffer. Using other diluents may result in wrong results.
7.Cross reactions maybe exist due to the N protein in SARS has a high homology with the SARS-CoV-2, especially in high titer.
Post time: Jan-13-2023
 中文网站
中文网站
