During the PCR reaction, some interfering factors are often encountered.
Due to the very high sensitivity of PCR, contamination is considered to be one of the most important factors affecting PCR results and can produce false positive results.
Equally critical are the various sources that lead to false-negative results. If one or more essential parts of the PCR mixture or the amplification reaction itself are inhibited or interfered with, the diagnostic assay can be hindered. This can lead to reduced efficiency and even false negative results.
In addition to inhibition, loss of target nucleic acid integrity may occur due to shipping and/or storage conditions prior to sample preparation. In particular, high temperatures or inadequate storage may lead to damage of cells and nucleic acids. Cell and tissue fixation and paraffin embedding are well known causes of DNA fragmentation and a persistent problem (see Figures 1 and 2). In these cases, even optimal isolation and purification will not help.
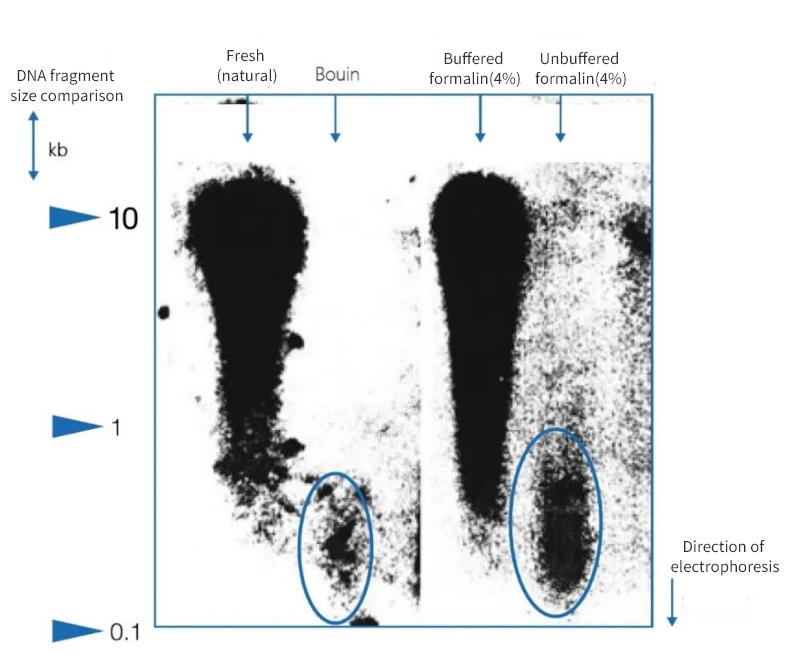
Figure 1 | Effect of immobilization on DNA integrity
Agarose gel electrophoresis showed that the quality of the DNA isolated from paraffin sections of autopsies varied considerably. DNA of different average fragment lengths was present in the extracts depending on the fixation method. DNA was preserved only when fixed in native frozen samples and in buffered neutral formalin. The use of a strongly acidic Bouin fixative or unbuffered, formic acid-containing formalin resulted in a significant loss of DNA. The remaining fraction is highly fragmented.
On the left, the length of fragments is expressed in kilobase pairs (kbp)
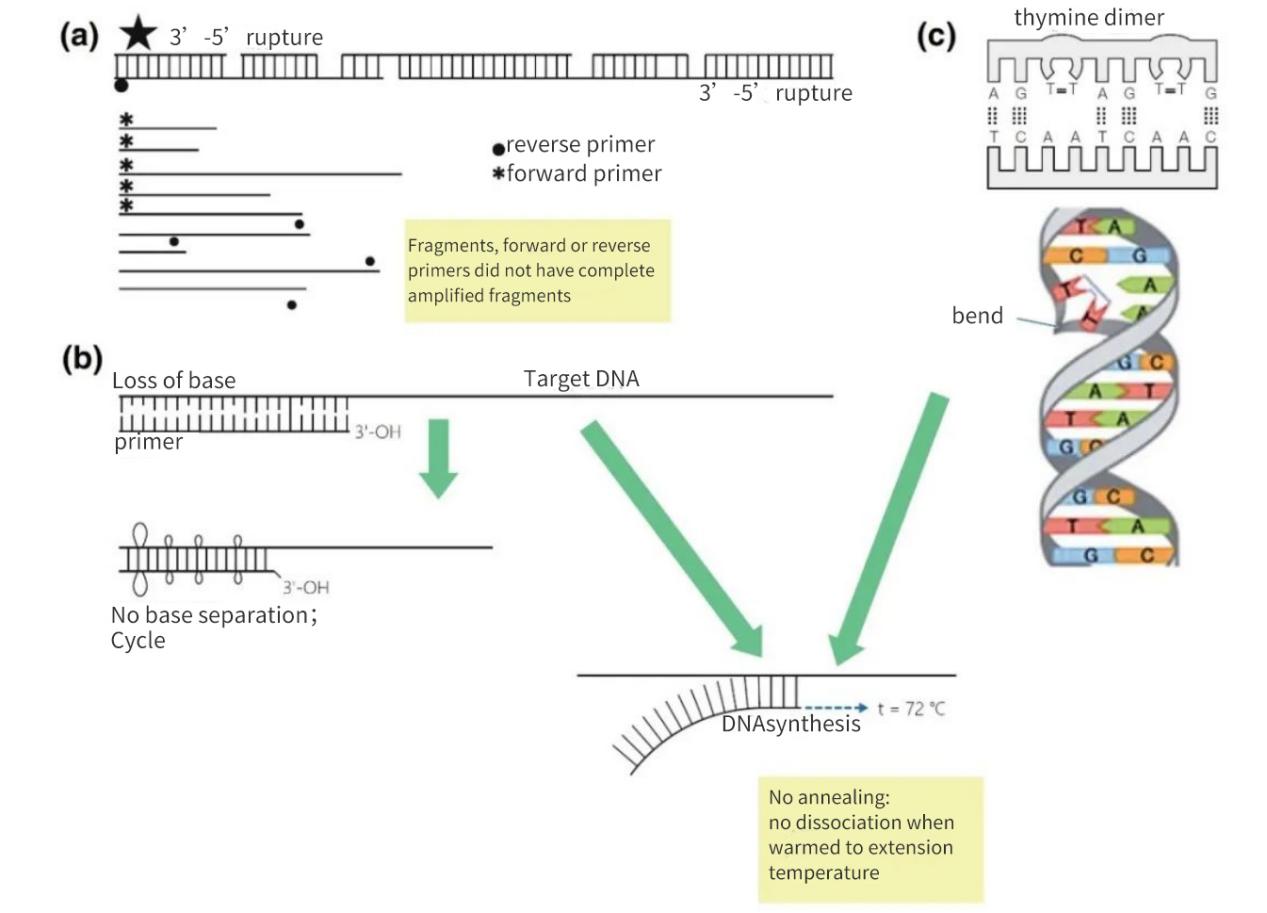
Figure 2 | Loss of integrity of nucleic acid targets
(a) A 3′-5′ gap on both strands will result in a break in the target DNA. synthesis of DNA will still occur on the small fragment. However, if a primer annealing site is missing on the DNA fragment, only linear amplification occurs. In the most favorable case, the fragments may resaturate each other, but the yields will be small and below detection levels.
(b) Loss of bases, mainly due to depurination and thymidine dimer formation, leads to a decrease in the number of H-bonds and a decrease in Tm. During the elongated warming phase, the primers will melt away from the matrix DNA and will not anneal even under less stringent conditions.
(c) Adjacent thymine bases form a T-T dimer.
Another common problem that often occurs in molecular diagnostics is the less-than-optimal release of target nucleic acids compared to phenol-chloroform extraction. In extreme cases, this can be associated with false negatives. Much time can be saved by boiling lysis or enzymatic digestion of cell debris, but this method often results in low PCR sensitivity due to insufficient nucleic acid release.
Inhibition of polymerase activity during amplification
In general, inhibition is used as a container concept to describe all factors that lead to suboptimal PCR results. In a strictly biochemical sense, inhibition is limited to the activity of the enzyme, i.e., it reduces or prevents substrate-product conversion through interaction with the active site of the DNA polymerase or its cofactor (e.g., Mg2+ for Taq DNA polymerase).
Components in the sample or various buffers and extracts containing reagents can directly inhibit the enzyme or trap its cofactors (e.g. EDTA), thereby inactivating the polymerase and in turn leading to decreased or false negative PCR results.
However, many interactions between reaction components and target-containing nucleic acids are also designated as ‘PCR inhibitors’. Once the integrity of the cell is disrupted by isolation and the nucleic acid is released, interactions between the sample and its surrounding solution and solid phase can occur. For example, ‘scavengers’ can bind single- or double-stranded DNA through non-covalent interactions and interfere with isolation and purification by reducing the number of targets that eventually reach the PCR reaction vessel.
In general, PCR inhibitors are present in most body fluids and reagents used for clinical diagnostic tests (urea in urine, hemoglobin and heparin in blood), dietary supplements (organic components, glycogen, fat, Ca2+ ions) and components in the environment (phenols, heavy metals)
|
Inhibitors |
Source |
|
Calcium ions |
Milk, bone tissue |
|
Collagen |
Tissue |
|
Bile salts |
Feces |
|
Hemoglobin |
In blood |
|
Hemoglobin |
Blood samples |
|
Humic acid |
Soil, plant |
|
Blood |
Blood |
|
Lactoferrin |
Blood |
|
(European) melanin |
Skin, hair |
|
Myoglobin |
Muscle tissue |
|
Polysaccharides |
Plant, feces |
|
Protease |
Milk |
|
Urea |
Urine |
|
Mucopolysaccharide |
Cartilage, mucous membranes |
|
Lignin, cellulose |
Plants |
More prevalent PCR inhibitors can be found in bacteria and eukaryotic cells, non-target DNA, DNA-binding macromolecules of tissue matrices and laboratory equipment such as gloves and plastics. Purification of nucleic acids during or after extraction is the preferred method for removing PCR inhibitors.
Today, various automated extraction equipment can replace many manual protocols, but 100% recovery and/or purification of targets has never been achieved. Potential inhibitors may still be present in the purified nucleic acids or may have already taken effect. Different strategies exist to reduce the impact of inhibitors. The choice of the appropriate polymerase can have a significant impact on inhibitor activity. Other proven methods to reduce PCR inhibition are increasing polymerase concentration or applying additives such as BSA.
Inhibition of PCR reactions can be demonstrated by the use of internal process quality control (IPC).
Care must be taken to remove all reagents and other solutions in the extraction kit, such as ethanol, EDTA, CETAB, LiCl, GuSCN, SDS, isopropanol and phenol, from the nucleic acid isolate by a thorough washing step. Depending on their concentration, they may activate or inhibit PCR.
Post time: May-19-2023
 中文网站
中文网站
