Recently, JAMA Oncology (IF 33.012) published an important research result [1] by the team of Prof. Cai Guo-ring from Cancer Hospital of Fudan University and Prof. Wang Jing from Renji Hospital of Shanghai Jiao Tong University School of Medicine, in collaboration with KUNYUAN BIOLOGY: “Early Detection of Molecular Residual Disease and Risk Stratification for Stage I to III Colorectal Cancer via Circulating Tumor DNA Methylation and Risk Stratification)”. This study is the first multicenter study in the world to apply PCR-based blood ctDNA multigene methylation technology for colorectal cancer recurrence prediction and recurrence monitoring, providing a more cost-effective technical pathway and solution compared with existing MRD detection technology methods, which is expected to greatly improve the clinical use of colorectal cancer recurrence prediction and monitoring, and significantly improve patient survival and quality of life . The study was also highly evaluated by the journal and its editors, and was listed as a key recommendation paper in this issue, and Professor Juan Ruiz-Bañobre from Spain and Professor Ajay Goel from the United States were invited to review it. The study was also reported by GenomeWeb, a leading biomedical media in the United States.

Colorectal cancer (CRC) is a common malignant tumor of the gastrointestinal tract in China. 2020 International Agency for Research on Cancer (IARC) data show that 555,000 new cases in China account for about 1/3 of the world, with the incidence rate jumping to the second place of common cancers in China; 286,000 deaths account for about 1/3 of the world, ranking as the fifth most common cause of cancer deaths in China. The fifth cause of death in China. It is noteworthy that among the diagnosed patients, the TNM stages I, II, III and IV are 18.6%, 42.5%, 30.7% and 8.2% respectively. More than 80% of the patients are in the middle and late stages, and 44% of them have simultaneous or heterochronic distant metastases to liver and lung, which seriously affect the survival period, endanger the health of our residents and cause heavy social and economic burden. According to the statistics of the National Cancer Center, the average annual increase in the cost of colorectal cancer treatment in China is about 6.9% to 9.2%, and the personal health expenditure of patients within one year of diagnosis can take up 60% of the family income. Cancer patients are suffering from the disease and also under great economic pressure [2].
Ninety percent of colorectal cancer lesions can be removed surgically, and the earlier the tumor is detected, the higher the five-year survival rate after radical surgical resection, but the overall recurrence rate after radical resection is still about 30%. The five-year survival rates of colorectal cancer in the Chinese population are 90.1%, 72.6%, 53.8% and 10.4% for stages I, II, III and IV, respectively.
Minimal residual disease (MRD) is a major cause of tumor recurrence after radical treatment. In recent years, MRD detection technology for solid tumors has advanced rapidly, and several heavyweight observational and interventional studies have confirmed that postoperative MRD status can indicate the risk of postoperative recurrence of colorectal cancer. ctDNA testing has the advantages of being noninvasive, simple, rapid, with high sample accessibility and overcoming tumor heterogeneity.
The US NCCN guidelines for colon cancer and the Chinese CSCO guidelines for colorectal cancer both state that for postoperative recurrence risk determination and adjuvant chemotherapy selection in colon cancer, ctDNA testing can provide prognostic and predictive information to assist in adjuvant treatment decisions for patients with stage II or III colon cancer. However, most existing studies focus on ctDNA mutations based on high-throughput sequencing technology (NGS), which has a complex process, long lead time, and high cost [3], with a slight lack of generalizability and low prevalence among cancer patients.
In the case of stage III colorectal cancer patients, NGS-based ctDNA dynamic monitoring costs up to $10,000 for a single visit and requires a waiting period of up to two weeks. With the multigene methylation test in this study, ColonAiQ®, patients can have dynamic ctDNA monitoring at a tenth of the cost and get a report in as little as two days.
According to the 560,000 new cases of colorectal cancer in China each year, the clinical patients mainly with stage II-III colorectal cancer (the proportion is about 70%) have more urgent demand for dynamic monitoring, then the market size of MRD dynamic monitoring of colorectal cancer reaches millions of people each year.
It can be seen that the research results have important scientific and practical significance. Through large-scale prospective clinical studies, it has confirmed that PCR-based blood ctDNA multigene methylation technology can be used for colorectal cancer recurrence prediction and recurrence monitoring with both sensitivity, timeliness and cost-effectiveness, better enabling precision medicine to benefit more cancer patients. The study is based on ColonAiQ®, a multi-gene methylation test for colorectal cancer developed by KUNY, whose clinical application value in early screening and diagnosis has been confirmed by a central clinical study.
Gastroenterology (IF33.88), the top international journal in the field of gastrointestinal diseases in 2021, reported the multicenter research results of Zhongshan Hospital of Fudan University, Cancer Hospital of Fudan University and other authoritative medical institutions in conjunction with KUNYAN Biological, which confirmed the excellent performance of ColonAiQ® ChangAiQ® in early screening and early diagnosis of colorectal cancer, and initially explored the It also explores the potential application in the prognosis monitoring of colorectal cancer.
To further validate the clinical application of ctDNA methylation in risk stratification, guiding treatment decisions and early recurrence monitoring in stage I-III colorectal cancer, the research team included 299 patients with stage I-III colorectal cancer who underwent radical surgery and collected blood samples at each follow-up point (three months apart) within one week before surgery, one month after surgery, and in postoperative adjuvant therapy for dynamic blood ctDNA testing.
First, it was found that ctDNA testing could predict the risk of recurrence in colorectal cancer patients early, both preoperatively and early postoperatively. Preoperative ctDNA-positive patients had a higher probability of postoperative recurrence than preoperative ctDNA-negative patients (22.0% > 4.7%). Early postoperative ctDNA testing still predicted recurrence risk: one month after radical resection, ctDNA-positive patients were 17.5 times more likely to recur than negative patients; the team also found that combined ctDNA and CEA testing slightly improved performance in detecting recurrence (AUC=0.849), but the difference was not significant compared with ctDNA (AUC=0.839) testing alone The difference was not significant compared to ctDNA alone (AUC=0.839).
Clinical staging combined with risk factors is currently the main basis for risk stratification of cancer patients, and in the current paradigm, a large number of patients still recur [4], and there is an urgent need for better stratification tools as over-treatment and under-treatment coexist in the clinic. Based on this, the team classified patients with stage III colorectal cancer into different subgroups based on clinical recurrence risk assessment (high risk (T4/ N2) and low risk (T1-3N1)) and adjuvant treatment period (3/6 months). The analysis found that patients in the high-risk subgroup of ctDNA-positive patients had a lower recurrence rate if they received six months of adjuvant therapy; in the low-risk subgroup of ctDNA-positive patients, there was no significant difference between the adjuvant treatment cycle and patient outcomes; while ctDNA-negative patients had a significantly better prognosis than ctDNA-positive patients and a longer postoperative recurrence-free period (RFS); stage I and low-risk stage II colorectal cancer All ctDNA-negative patients had no recurrence within two years; therefore, the integration of ctDNA with clinical features is expected to further optimize risk stratification and better predict recurrence.
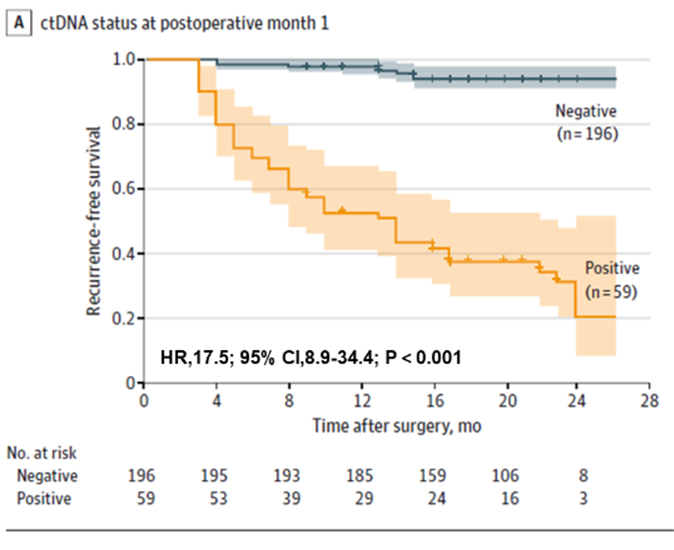
Figure 1. Plasma ctDNA analysis at POM1 for early detection of colorectal cancer recurrence
Further results of dynamic ctDNA testing showed that the risk of recurrence was significantly higher in patients with positive dynamic ctDNA testing than in patients with negative ctDNA during the disease recurrence monitoring phase after definitive treatment (after radical surgery + adjuvant therapy) (Figure 3ACD), and that ctDNA can indicate tumor recurrence up to 20 months earlier than imaging (Figure 3B), offering the possibility of early detection of disease recurrence and timely intervention.
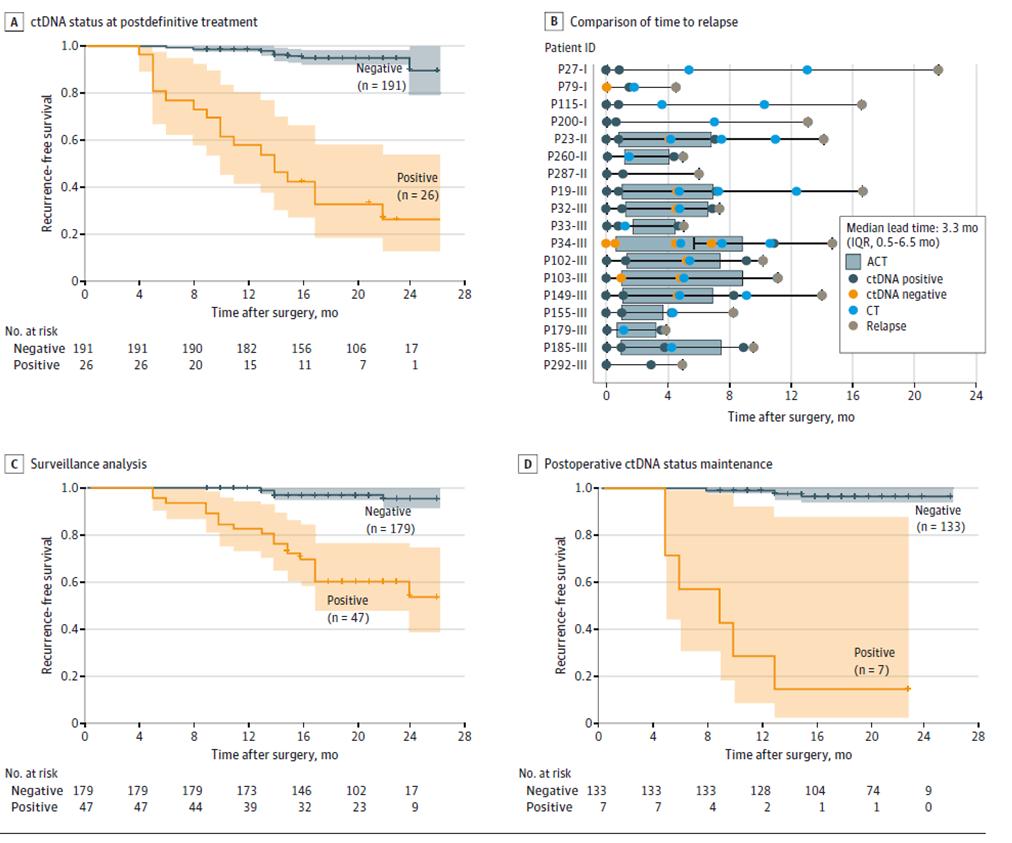
Figure 2. ctDNA analysis based on longitudinal cohort to detect colorectal cancer recurrence
“A large number of translational medicine studies in colorectal cancer lead the discipline, especially ctDNA-based MRD testing demonstrates great potential to enhance postoperative management of colorectal cancer patients by enabling recurrence risk stratification, guiding treatment decisions and early recurrence monitoring.
The advantage of choosing DNA methylation as a novel MRD marker over mutation detection is that it does not require whole genome sequencing screening of tumor tissues, is directly used for blood testing, and avoids false-positive results due to detection of somatic mutations originating from normal tissues, benign diseases, and clonal hematopoiesis.
This study and other related studies confirm that ctDNA-based MRD testing is the most important independent risk factor for recurrence of stage I-III colorectal cancer and can be used to help guide treatment decisions, including “escalation” and “downgrading” of adjuvant therapy MRD is the most important independent risk factor for recurrence after surgery for stage I-III colorectal cancer.
The field of MRD is rapidly evolving with a number of innovative, highly sensitive and specific assays based on epigenetics (DNA methylation and fragmentomics) and genomics (ultra-deep targeted sequencing or whole genome sequencing). We expect that ColonAiQ® continues to organize large-scale clinical studies and can become a new indicator of MRD testing that combines accessibility, high performance and affordability and can be widely used in routine clinical practice.”
References
[1] Mo S, Ye L, Wang D, Han L, Zhou S, Wang H, Dai W, Wang Y, Luo W, Wang R, Xu Y, Cai S, Liu R, Wang Z, Cai G. Early Detection of Molecular Residual Disease and Risk Stratification for Stage I to III Colorectal Cancer via Circulating Tumor DNA Methylation. JAMA Oncol. 2023 Apr 20.
[2] “The burden of colorectal cancer disease in the Chinese population: has it changed in recent years? , Chinese Journal of Epidemiology, Vol. 41, No. 10, October 2020.
[3] Tarazona N, Gimeno-Valiente F, Gambardella V, et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann Oncol. Nov 1, 2019;30(11):1804-1812.
[4] Taieb J, André T, Auclin E. Refining adjuvant therapy for non-metastatic colon cancer, new standards and perspectives. Cancer Treat Rev. 2019;75:1-11.
Post time: Apr-28-2023
 中文网站
中文网站
